Tested: Preparing for COVID-19
Tested: Preparing for COVID-19
EAST LANSING: As COVID-19 spreads from China, public health officials the world over mobilize, preparing for declaration of a global pandemic. “It's not so much a question of if this will happen anymore, but rather more a question of when this will happen,” said Anne Schuchat, principal deputy director of the US Centers for Disease Control.
Preparation includes a scramble to understand transmission patterns by developing and distributing accurate tests. Expanded testing may spike case totals, but early detection, isolation and treatment reduces the spread of the disease and fatalities.
The World Health Organization declared a global health emergency on January 30, urging a coordinated global response after reports of new coronavirus cases began in Wuhan and spread beyond China. A month later, more than 50 countries report confirmed cases – more than 78,000 in China, 1,200 in South Korea, 700 on a cruise ship, 400 in Italy, and more than 100 in Japan and Iran. WHO defines an epidemic as rapid spread of disease within a community or region, with reports of cases higher than expected. A pandemic is the spread of a new disease, not necessarily severe, across continents where immunity is lacking, such as HIV/AIDS and some strains of the flu. Research suggests that more than 80 percent of cases report mild symptoms.
WHO applies care in issuing declarations that can trigger tremendous social and economic disruptions – including panic buying, stock downturns, closed borders and discrimination targeting ethnic groups – and urged against restricting travel or trade with China.
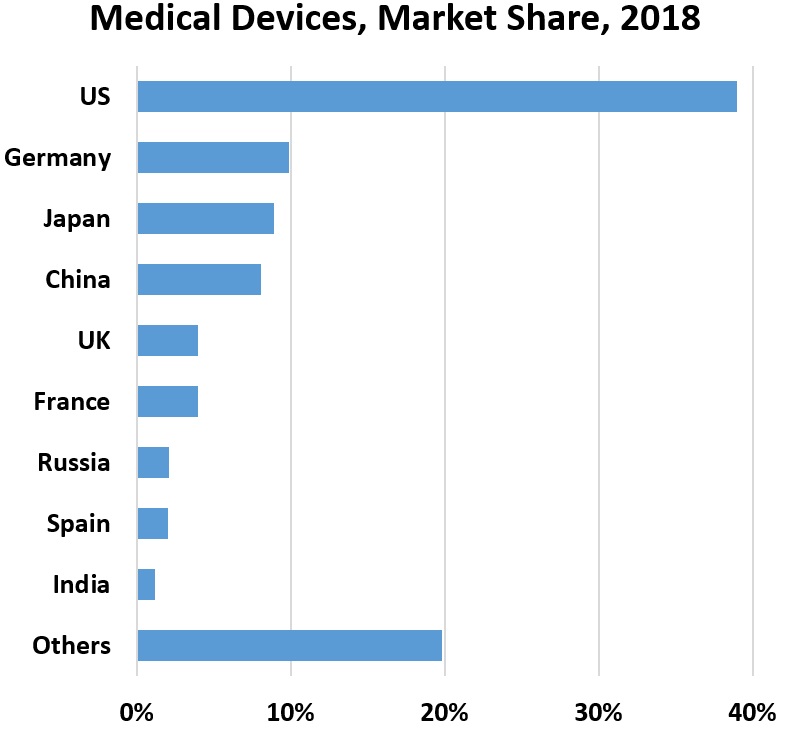
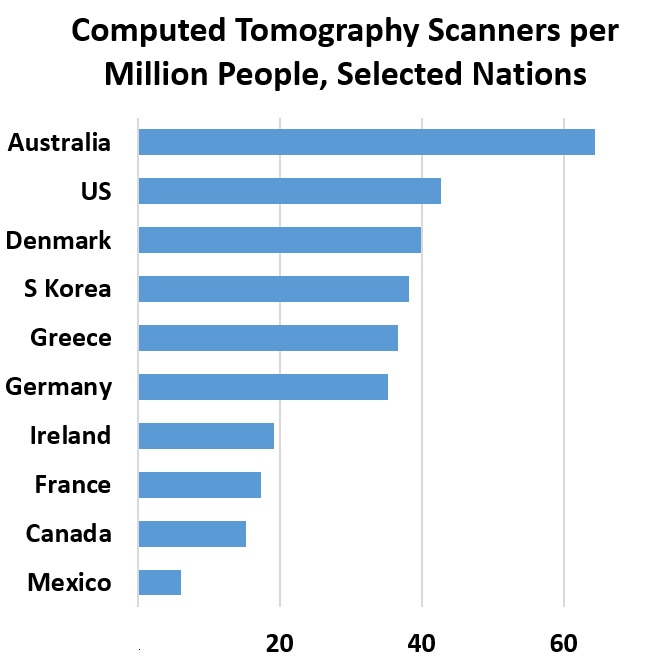
The world relies on China’s large pool of cases for research on treatment and testing. As diagnostic testing evolved, China revised methods for tallying confirmed cases. Not all clinics had access to tests, and for a week in February, totals relied on clinical diagnosis. Chinese researchers conducted a study of more than 1,000 patients, reporting that computed tomography scans outperform lab testing for COVID-19 diagnosis. The researchers recommend CT scans as the “primary tool for the current COVID-19 detection in epidemic areas.”
Chest CT scans cost about $1000 in the US and $140 in the Netherlands, reports Vox. Blood, nasal, mouth-swab and other rapid tests cost less.
Public health officials share findings on COVID-19 and governments decide on a range of strategies while approving diagnostic testing products and organizing delivery to local health departments. China quickly identified and released the genetic sequence for the coronavirus in mid-January. Soon afterward, “China's national institute of infectious diseases and a company in Jiangsu Province announced the joint development of a technology that can promptly determine whether the virus is present,” reports Nikkei Asian Review.
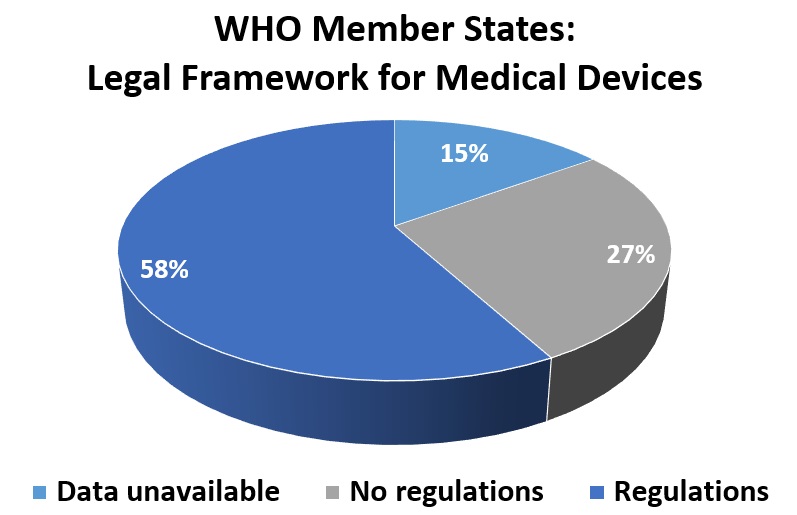
Developing and distributing new test kits is a challenge. In vitro diagnostic testing makes up a sliver of overall health care spending, for example, representing less than 1 percent in Europe for 2017. Developers must consider health care systems, logistics, regulations, supply chains, costs and political commitment. The tests must be tested themselves with live virus samples to assess sensitivity and accuracy. Diagnostic tests, categorized as medical devices, are “usually conducted in laboratories, private or public, equipped with appropriate and sometimes expensive instrumentation and staffed with trained and qualified personnel to perform the tests,” reports the National Center for Biotechnology Information. The goal is for point-of-care testing near patients for fast delivery of results to guide treatment decisions.
By mid-February, WHO appointed 16 referral laboratories for confirming new cases in China, Japan, Singapore, Australia, Thailand, India, the United States, South Africa, Senegal, Russia, Germany, the Netherlands, the United Kingdom and France. Another 150 laboratories have technology for identifying the virus, reports Reuters. Agencies in China, Germany, Hong Kong, Japan, Thailand and the United States have shared protocols for molecular assays online. WHO is in regular contact with researchers and manufacturers.
Japan has warned that distribution of fully tested kits could take at least three months. A test distributed to US labs in early February was reported as flawed, and local health departments wait for new kits. Simple tests tend to have less accuracy, warn developers.
Singapore identified links among cases by tracking patient movements and contacts and using a serological test from Duke-NUS Medical School. “The research team at Duke-NUS Medical School had earlier successfully cultured the COVID-19 virus in less than a week after Singapore confirmed its first COVID-19 case,” reports the Ministry of Health in Singapore. Researchers then used the virus and genetic material to develop a laboratory test to detect antibodies for contact tracing.
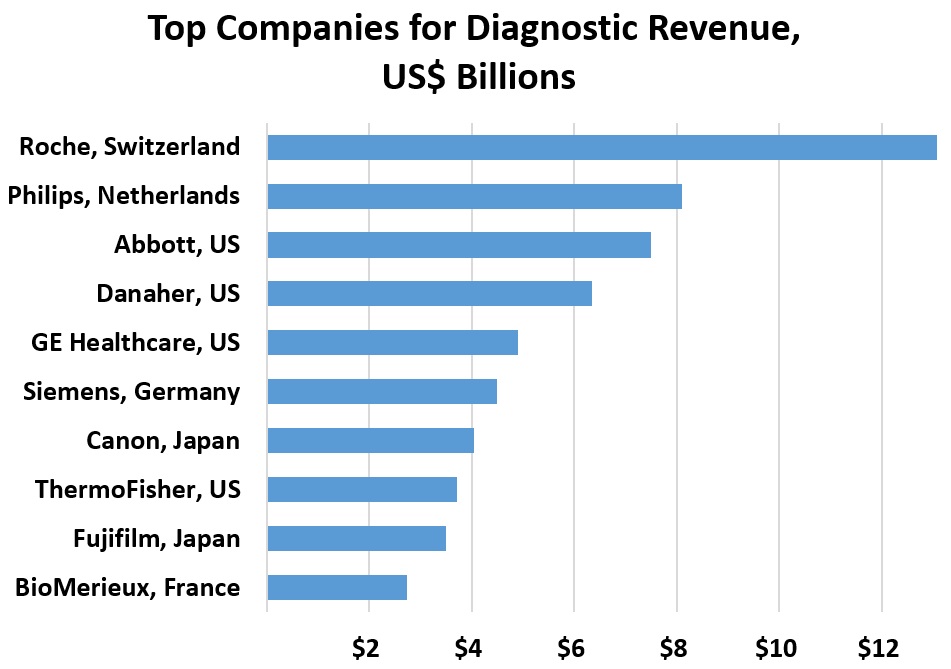
For now, the public health goal is to use limited tests for patients showing symptoms. In Japan, “guidelines say people should get tested if they have cold-like symptoms, a fever of 37.5 Celsius or extreme tiredness or breathing problems for four days or more.” Japan’s health minister reported an average of 900 tests per day even as hospitals turn people away. Efforts focus on people who have traveled to China or show symptoms. People with symptoms should contact health providers and otherwise stay at home.
WHO offers advice for selecting diagnostic tests, recommending certification and approval from European and US regulators and manufactured in facilities that meet conditions from the International Organization for Standardization.
In the United States about 40 labs besides the CDC have capability to test for the virus, with more coming on next week, reports CIDRAP News. Currently, hundreds of samples are tested with overnight results, explained Nancy Messonnier, MD, director of the CDC's National Center for Immunization and Respiratory Diseases. Six additional labs in the United States plan to start testing this week.
Surveillance systems for the flu and other viruses are in place, with local health teams ready to test and report results to the CDC. “Surveillance for the novel coronavirus will take advantage of all of these existing methods and platforms,” reports Nicole Wetsman for Verge. She explains that CDC expects local health labs to test any samples from patients showing symptoms that screen negative for influenza. Labs can also test combined samples to speed the process of pinpointing coronavirus locations.

WHO expresses concern about the coronavirus in Africa, with Egypt, Algeria and Nigeria each reporting one case. “The threats posed by COVID-19 has cast a spotlight on the shortcomings in health systems in the African Region,” said Matshidiso Moeti, WHO Regional Director for Africa, in a statement. Africa’s investments in preparation for the flu and Ebola, including laboratories, support the coronavirus response.
WHO has encouraged rapid technology transfer for early diagnosis to help vulnerable populations since 2011. “High-quality tests for infectious diseases are readily available in most developed countries; however, the situation is very different in developing countries, where the cost of imported IVD technology is frequently too high for the public sector,” notes a WHO report. “Transfer of health-related technologies has been credited with the potential to build health security, increase reliability of supply, decrease reliance on imports, lead to lower prices, and encourage development and production that is more suitable for local health needs.”
Another challenge in developing diagnostic products: WHO warns tests can have short lifespans, as diseases subside or new products arrive to market.
Susan Froetschel is editor of YaleGlobal Online.
The number of US labs allowed to test for COVID-19, the number of countries with cases and the number African nations with one case were last updated February 28.
Reference to the China Daily report on costs of PET CT scans was removed. See the reader's comment below.
Comments
The cited webpage about CT scan pricing in China is actually talking about PET/CT. That's why the pricing is so expensive "7000 yuan, about $950". According to a spreadsheet found on Beijing government's website, which hospitals need to follow (http://ybj.beijing.gov.cn/cxfw/ywmlcx/ylfy/, http://ybj.beijing.gov.cn/cxfw/ywmlcx/ylfy/201811/P020190619316803916484...), chest CT (line 150 in the third sheet of the file, ID: EBAJT001) is 135 yuan or about $19 in Beijing, which looks about right. Actually, even MRI is only about 400 to 600 yuan in China.